Towards modulating the gut microbiota to enhance the efficacy of immune-checkpoint inhibitors
Abstract
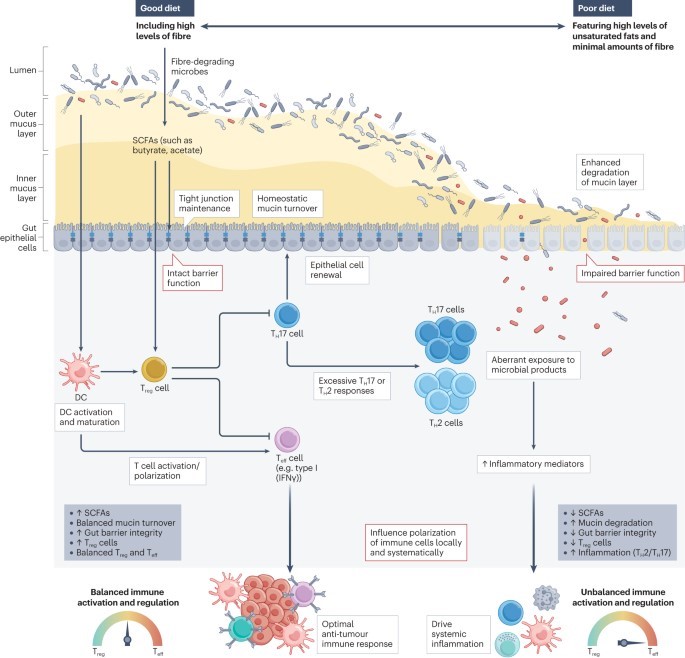
The gut microbiota modulates immune processes both locally and systemically. This includes whether and how the immune system reacts to emerging tumours, whether antitumour immune responses are reactivated during treatment with immune-checkpoint inhibitors (ICIs), and whether unintended destructive immune pathologies accompany such treatment.
Advances over the past decade have established that the gut microbiota is a promising target and that modulation of the microbiota might overcome resistance to ICIs and/or improve the safety of treatment. However, the specific mechanisms through which the microbiota modulates antitumour immunity remain unclear.
Understanding the biology underpinning microbial associations with clinical outcomes in patients receiving ICIs, as well as the landscape of a ‘healthy’ microbiota would provide a critical foundation to facilitate opportunities to effectively manipulate the microbiota and thus improve patient outcomes. In this Review, we explore the role of diet and the gut microbiota in shaping immune responses during treatment with ICIs and highlight the key challenges in attempting to leverage the gut microbiome as a practical tool for the clinical management of patients with cancer.
Key points
-
Growing evidence supports a role for the gut microbiota in shaping both antitumour immune responses and the development of toxicities during treatment with immune-checkpoint inhibitors (ICIs).
- Targeting the gut microbiota provides a potentially powerful tool to overcome resistance to ICIs and/or to reduce the risk of severe toxicities.
- Interindividual microbial heterogeneity poses a major challenge to the study of the microbiota across human populations and to the translation of microbial findings into the clinic.
- Interventions designed to modulate the microbiota could have profound effects in augmenting the effectiveness of ICIs in a subset of patients, although this strategy is unlikely to be uniformly effective; identifying which patients will benefit is an important step.
-
A personalized approach guided by a patient’s baseline gut microbiota, systemic immune parameters and tumour characteristics will be important in order to optimally target the microbiota and thus improve the outcomes of patients receiving ICIs.