Melanoma Institute Australia’s leading experts and early career researchers have shared the world stage at the annual European Society for Medical Oncology (ESMO) Congress in Barcelona.
The ESMO Congress attracts thousands of researchers and clinicians discussing latest research and treatment breakthroughs across all cancers.
MIA Co-Medical Director, Prof Georgina Long AO, and others from the MIA team including A/Prof Alex Menzies, A/Prof Ines Da Silva and A/Prof Alexander van Akkooi, PhD candidate and medical oncologist Dr Jorja Braden and medical oncology Fellow Dr Andrea Boutros, all presented data at the Congress.
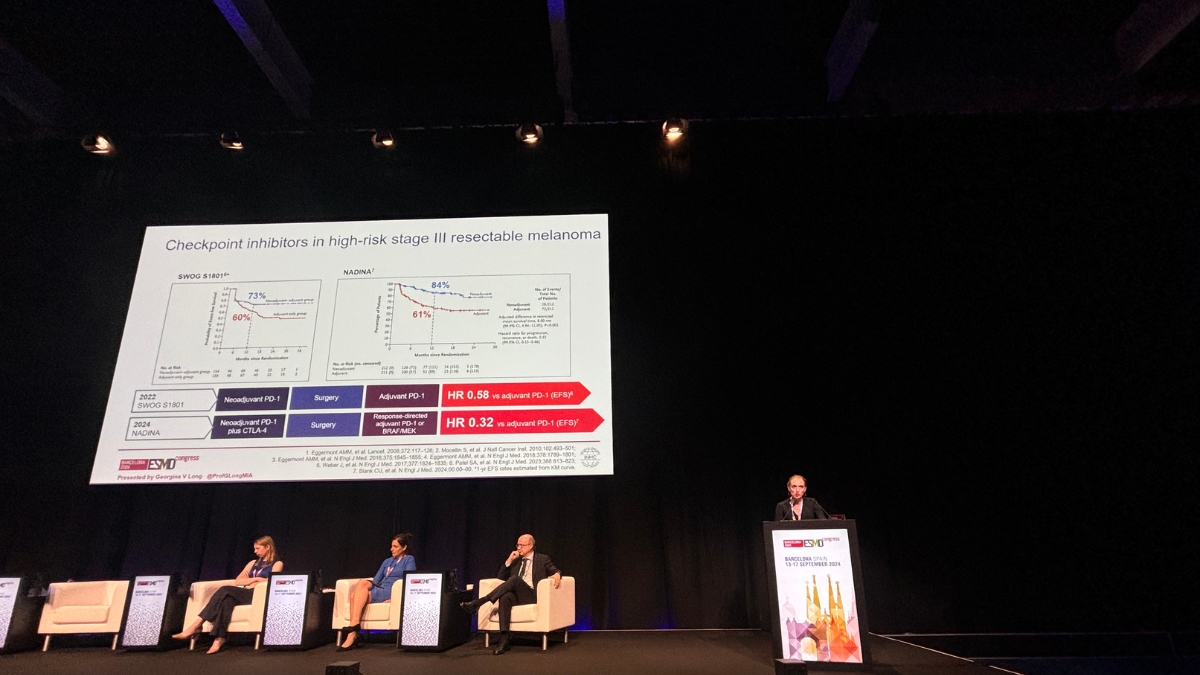
Professor Long leads an ongoing collaborative study from the International Neoadjuvant Melanoma Consortium (INMC), of which MIA and Prof Long are amongst the founding members. This study is the world’s largest pooled analysis of melanoma patients treated with neoadjuvant, or pre-surgery, drug therapy, which is now the standard of care for resectable stage III and advanced melanoma. Prof Long provided ESMO delegates with a survival update of an expanded cohort of 818 patients.
‘The pooled analysis showed neoadjuvant combination immune checkpoint inhibitors provide an unprecedented and lasting survival benefit to this group of patients, over and above other drug therapies,’ – Prof Georgina Long
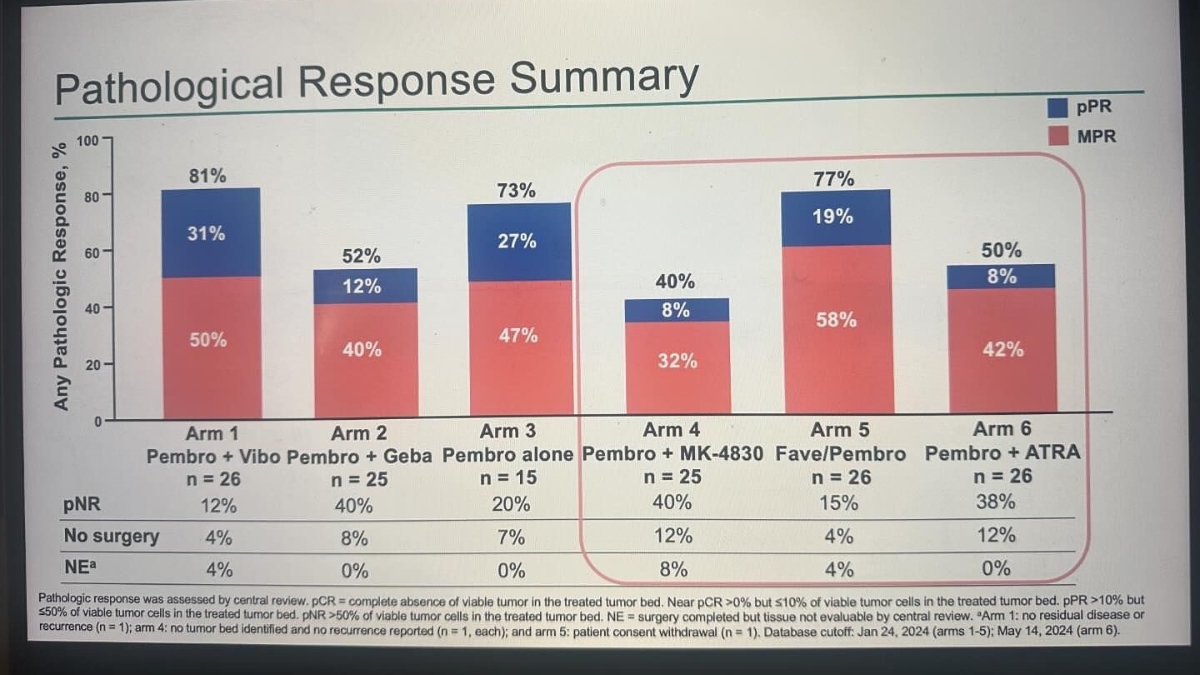
A/Prof Alex Menzies delivered an oral presentation on behalf of the Umbrella O2C study investigators evaluating neoadjuvant (pre-surgery) immunotherapy pembrolizumab with or without investigational agents followed by adjuvant (post-surgery) pembrolizumab for Stage IIIB-D melanoma. He told delegates all arms had manageable safety and promising antitumor activity in these patients, with further investigations warranted.
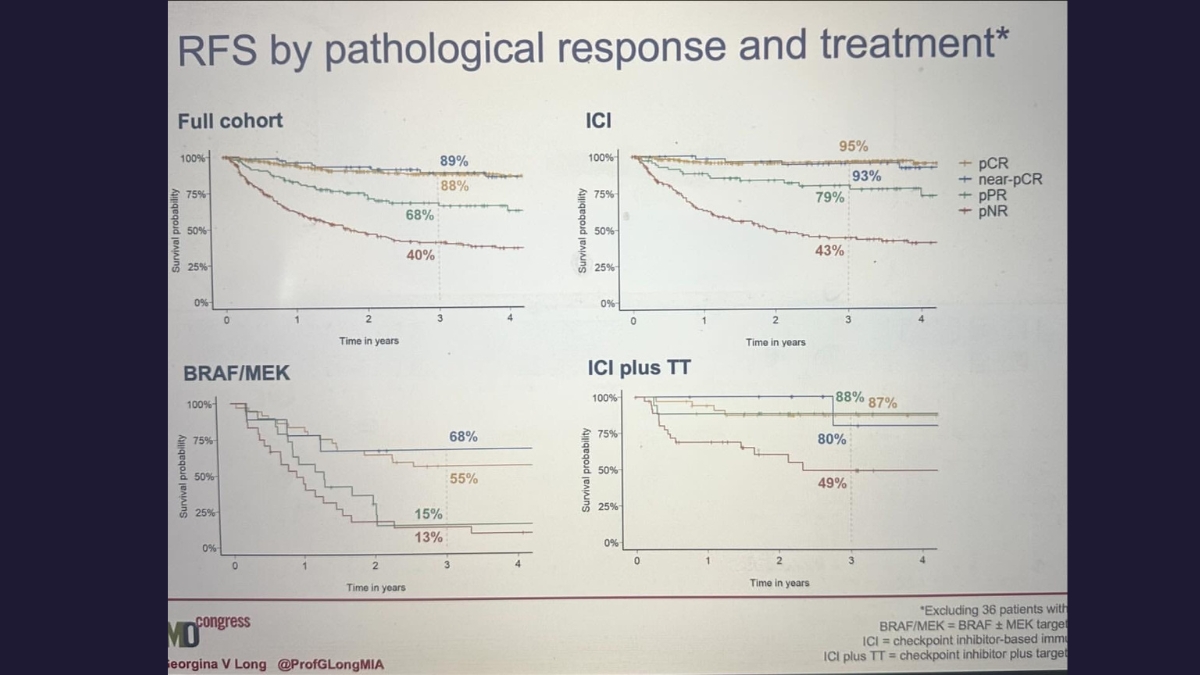
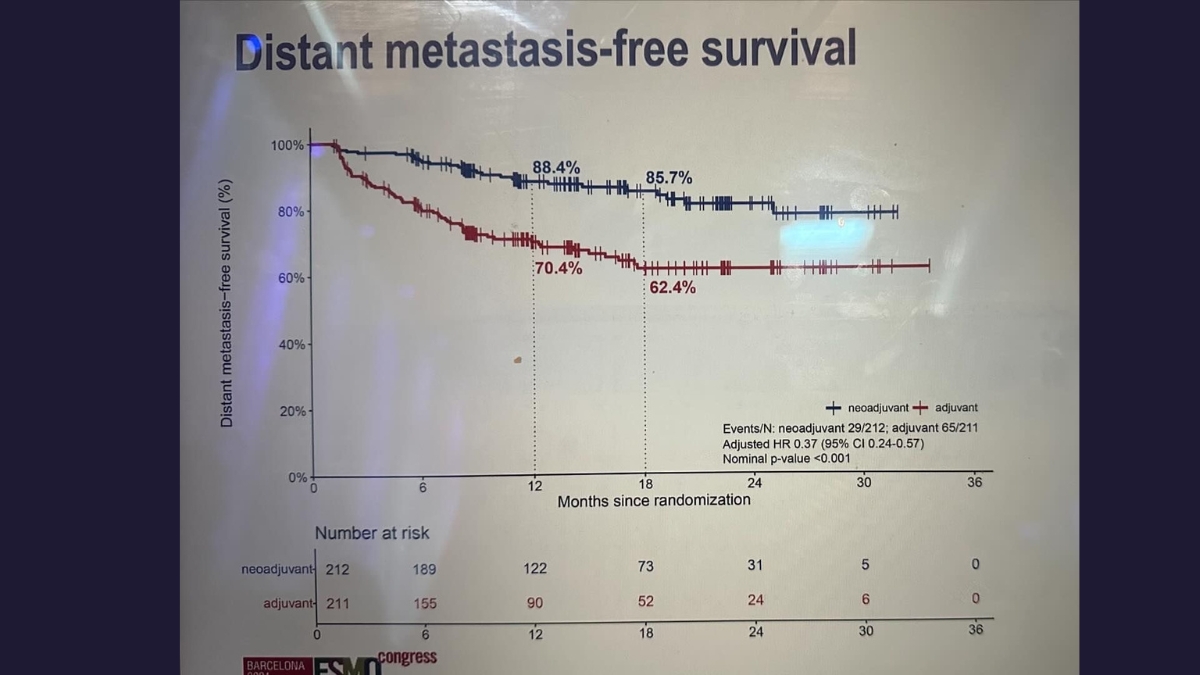
Results from the practice-changing NADINA clinical trial, the first phase 3 trial in oncology evaluating a neoadjuvant regimen consisting of only immunotherapy, were also relayed to delegates. NADINA was a collaboration between MIA and The Netherlands Cancer Institute, which was opened across the globe including in eight Australian centres.
It compared neoadjuvant treatment with combination immunotherapy (ipilimumab and nivolumab) to the current standard adjuvant (post-surgery) approach with single agent immunotherapy (nivolumab). Results showed estimated 12 month event-free survival (EFS) rates of 83.7% in the neoadjuvant arm vs 57.2% in the adjuvant arm, and that neoadjuvant combination immunotherapy significantly decreased the risk of distant spread.
‘This has the power to transform cancer treatment across the world,’ said Prof Long who designed the study with Professor Christian Blank, and led the Australian arm of the trial. ‘Combination neoadjuvant, or pre-surgery, immunotherapy should now be considered a new standard of treatment for higher-risk Stage III melanoma, and should also now be clinically evaluated for use across the wider oncology field.’
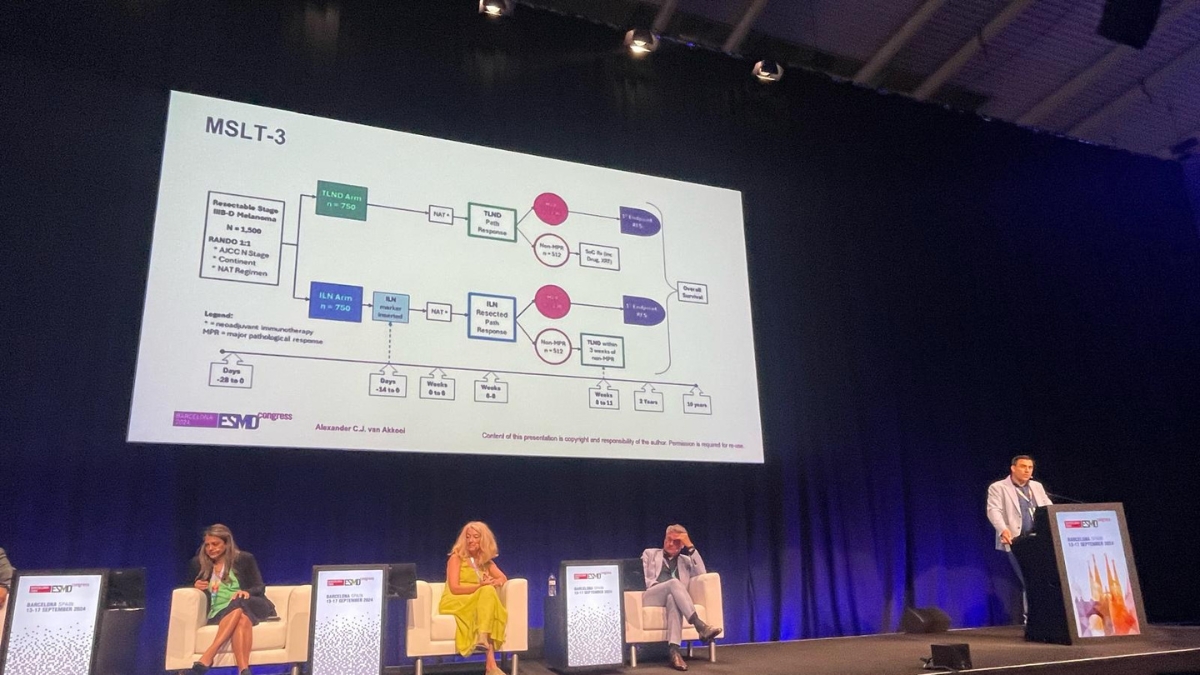
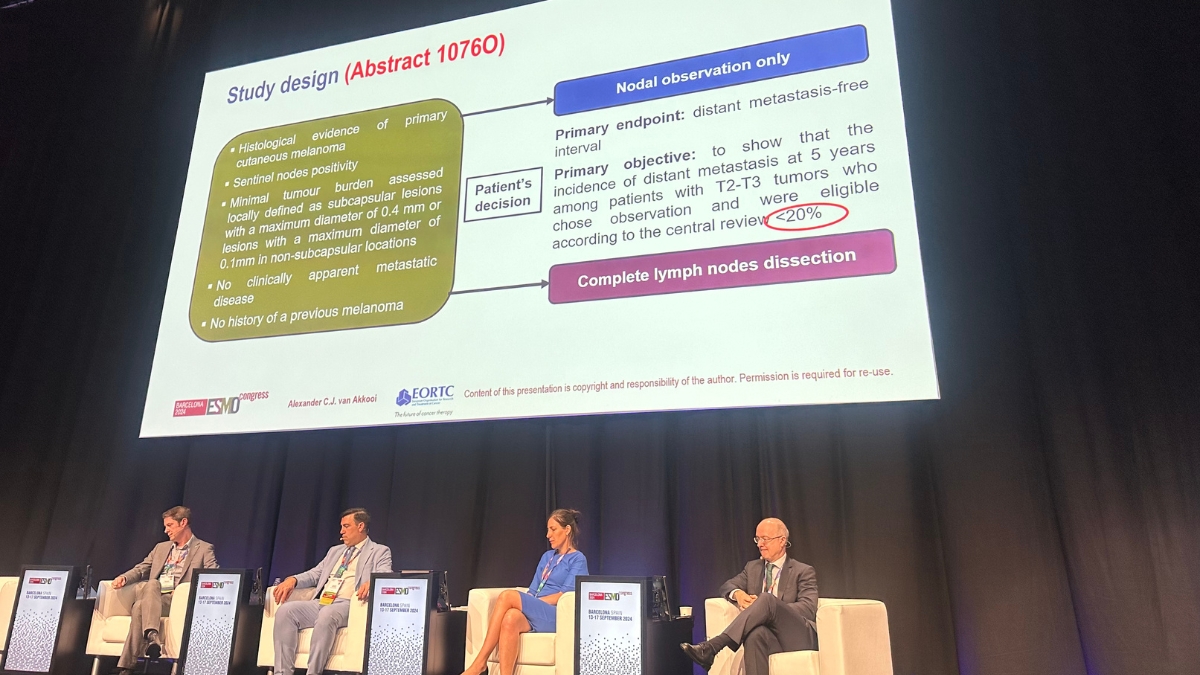
MIA Surgical Oncologist, A/Prof Alexander van Akkooi, featured in a number of sessions including those related to the importance of understanding the burden of melanoma in the sentinel lymph node, particularly in early stage melanoma.
‘The trial looked at the outcome of patients with low volume disease in the sentinel node. It confirmed the excellent prognosis of these patients and that volume of disease should be considered when making treatment decisions,’ – A/Prof Alex van Akkooi.
10 year follow up results from the Phase III KEYNOTE-006 study were also presented at ESMO, coinciding with the release of a paper in the Annals of Oncology with Prof Long as first author. Long-term follow-up data continued to show overall survival benefit with pembrolizumab vs ipilimumab in patients with advanced melanoma.
Prof Long’s final ESMO presentation was a mini-oral detailing 3-year results from the CheckMate 76K trial of adjuvant nivolumab vs placebo in early stage melanoma patients. At a median follow-up of 34.2 months, adjuvant nivolumab continued to demonstrate benefit for patients with resected Stage IIB/C melanoma.
MIA driven research also featured in presentations at ESMO from A/Prof Ines da Silva as well as PhD candidate Dr Jorja Braden and Fellow Dr Andrea Boutros.
One of A/Prof da Silva’s two posters detailed a predictive tool for neoadjuvant therapy response. Neoadjuvant immunotherapy with anti-PD-1 (PD1) is now a standard of care for patients with resectable stage IIIB–D melanoma, but although pathological response is predictive of recurrence, this variable alone cannot accurately identify those patients who will recur.
The assessment tool takes into account a patient’s demographic, disease characteristics, and pathological and imaging data.
‘The NeoRisk tool can accurately predict recurrence after neoadjuvant immunotherapy,’ A/Prof da Silva told delegates, ‘and will be critical in helping tailor post-surgery treatment and radiological surveillance intervals, based on the predicted risk of recurrence for individual patients.’
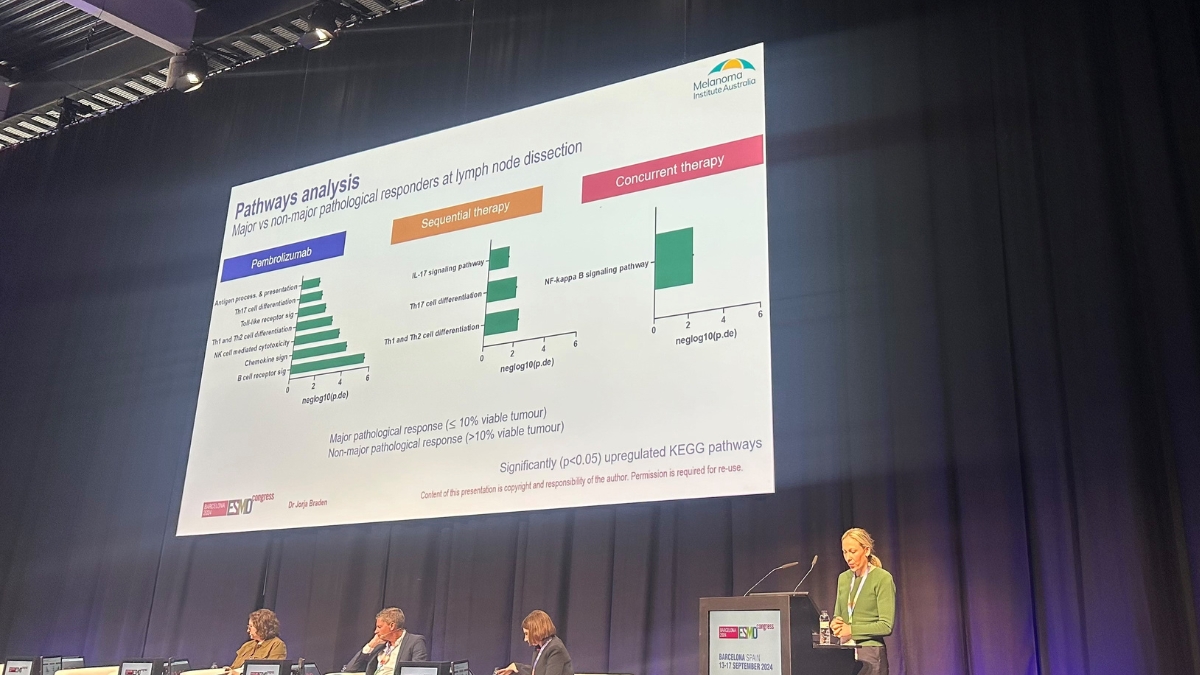
MIA PhD candidate Dr Jorja Braden delivered a prestigious mini-oral presentation on the translational aspects of the NeoTrio clinical trial. The neoadjuvant trial was also opened by MIA at Peter MacCallum Cancer Centre in Melbourne and Westmead Hospital in Sydney. Dr Braden’s presentation included detailing concurrent dabrafenib and trametinib targeted therapy with anti-PD-1 increasing B cell signalling and inflammatory pathways more effectively than when given sequentially or with anti-PD-1 alone.
MIA Medical Oncology Fellow Dr Andrea Boutros also provided updated results from the MATCH MEL trial, which involved molecular profiling and matched targeted therapy for patients with advanced melanoma.
‘The breadth of MIA presentations at ESMO is indicative of the depth of our research across all stages of disease, and the critical input also coming from early-career researchers and clinicians,’
‘I was proud to see MIA’s experts once again leading discussion about melanoma research and treatment on the world stage, particularly our PhD candidates and Fellows who are vital to our future efforts to reach zero deaths from melanoma,’ Professor Long said.