The U.S. Food and Drug Administration (FDA) has approved a new combination immunotherapy as first line treatment for unresectable advanced melanoma.
The approval of the first LAG-3-blocking antibody combination of nivolumab and relatlimab follows the international RELATIVITY-047 clinical trial, for which Melanoma Institute Australia’s Co-Medical Director Professor Georgina Long AO was senior lead author and MIA was a leading contributor of patients.
‘Australian researchers and patients were critical to trialling this new treatment for advanced melanoma patients, which has now been approved by the FDA,’ Professor Long said. ‘The next step is Therapeutic Goods Administration approval and consideration by the Pharmaceutical Benefits Advisory Committee to be funded in Australia.’
Trial results showed that in previously untreated advanced melanoma patients, combining relatlimab (a new immune checkpoint inhibitor that targets LAG-3) with nivolumab (an immune checkpoint inhibitor targeting the PD-1 protein) doubled the progression free survival time compared to the use of nivolumab alone. At one year, almost 50% of patients on the combination therapy had no disease progression, whereas nearly two-thirds of patients on the single therapy had progressed. Importantly, the combined therapy was also far less toxic to patients.
‘Approval of this new combination immunotherapy for advanced melanoma patients will improve outcomes and likely impact cancer treatment globally,’ Professor Long added. ‘This shows the power of research and the vital role that MIA continues to play in ground-breaking clinical trials which are changing clinical practice globally. Our mission is zero deaths from melanoma, and we are making inroads.’
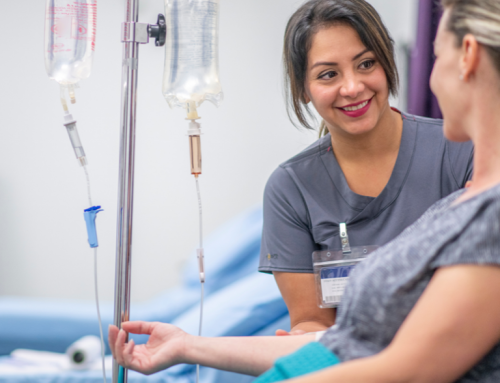
MIA patient Andrew Bennett, who was part of the RELATIVITY-047 trial is pictured.
Watch the story about Andrew Bennett and this breakthrough clinical trial, as featured on Channel 9 news last year when the research paper was published:





Leave A Comment